Sophisticated Inspection, Finishing, and Warehouse Center
GRAM’s world-class finishing center includes the latest design and technology for labeling, packaging, and inspection. Our vast inspection capabilities and experience with difficult-to-inspect products support pharmaceutical project needs. The inspection, finishing, and warehouse center is 3.4 miles from the Gerald R. Ford International Airport and under 20 minutes from GRAM’s downtown campus.
On February 13, 2024, we announced the completion of our 90,000 sq. ft. finishing center expansion. The facility totals 200,000 sq. ft. of sophisticated inspection, packaging, testing, and warehouse capabilities.
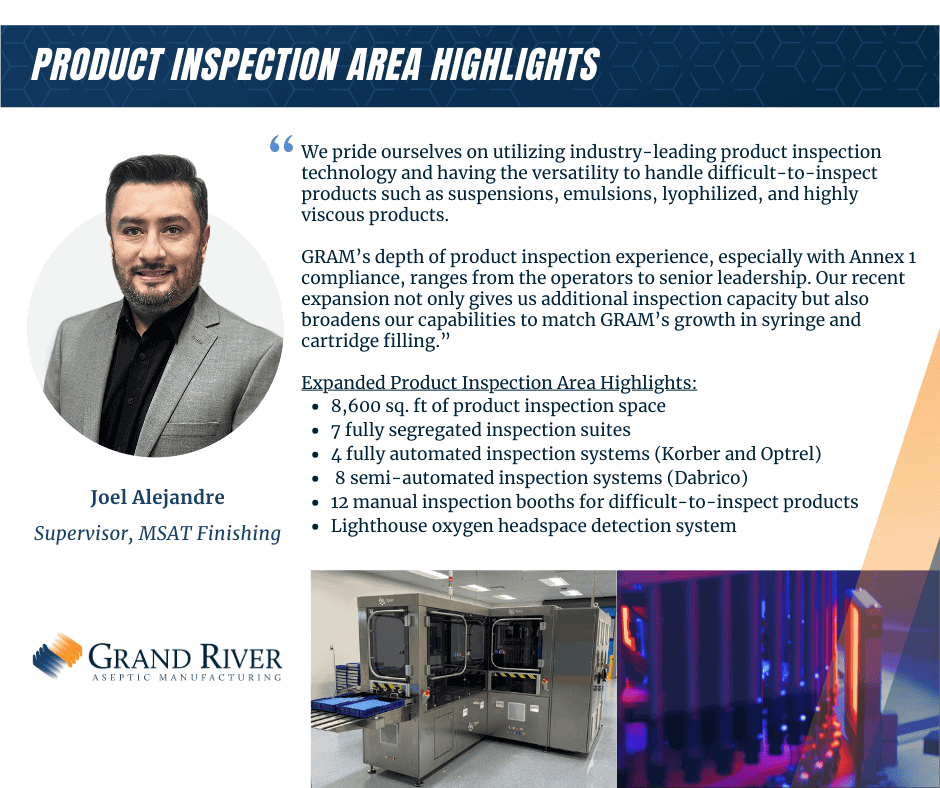
Inspection
- Two fully-automated Stevanato Optrel systems
- Innovative inspection algorithms ensure high detection rates
- Reduced false rejection integrating high voltage leak detection
- Capable of inspecting water-like, viscous, suspension, and lyophilized products
- Fully automated Korber Seidenator VI 40S vial inspection machine
- Fully automated Korber syringe inspection machine
- Eight semi-automated Dabrico units
- Twelve manual inspection booths
- Lighthouse Headspace Oxygen Analyzer (HSO)
- Provides automated, 100% inspection of headspace oxygen in sealed parenteral containers
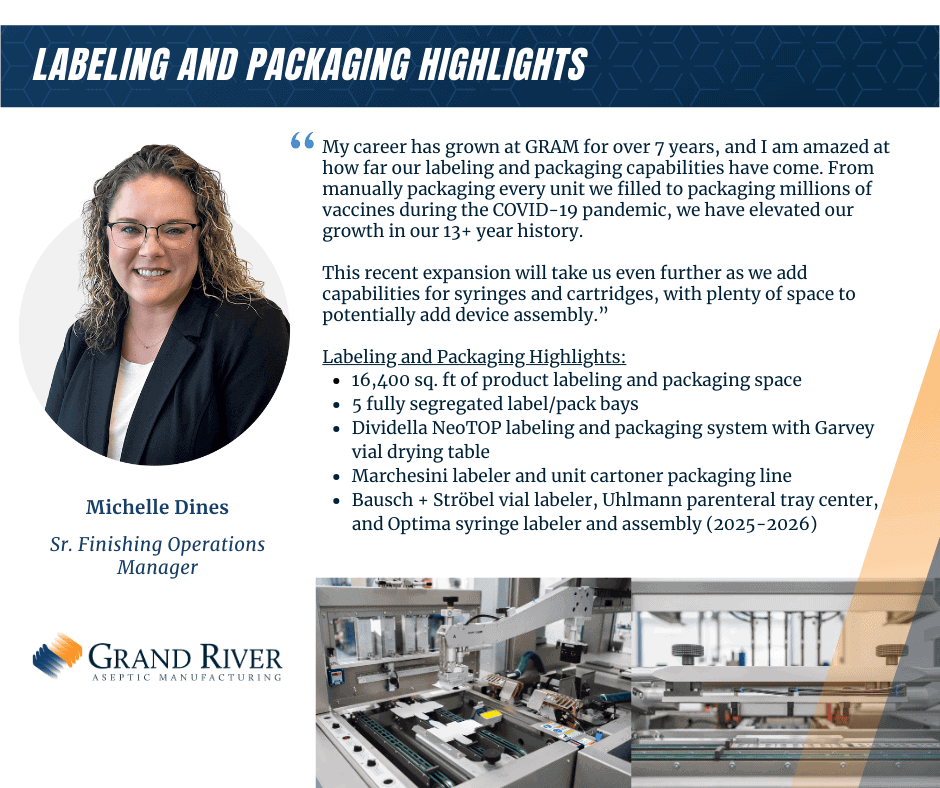
Labeling and Packaging with Serialization and Full Aggregation
- Dividella packaging system with drying table, labeler, cartoner, and case packer
- Temperature-controlled products can be processed quickly
- Check weighing and Tamper Evident capabilities to meet regulatory requirements
- Marchesini labeling and packaging with cartoner, check weighed, labeler, bundler, and case packer
- Lines interact with SEA Vision and TraceLink with four levels of serialization
- Bausch and Stroebel ESA1025 Labeler
- Uhlmann Parenteral Tray Center PTC 200
- Modular format that can package vials, syringes, cartridges, or combinations of vials and syringes
Controlled Temperature Storage
- Controlled room temperature storage (20-25°C)
- ~20,000 sq. ft. of finished product storage
- ~30,000 sq. ft. of material storage
- Cold and frozen storage
- +2°C to +8°C
- 2-8°C and -20°C
- GRAM company vehicles to support temperature-controlled material transfers
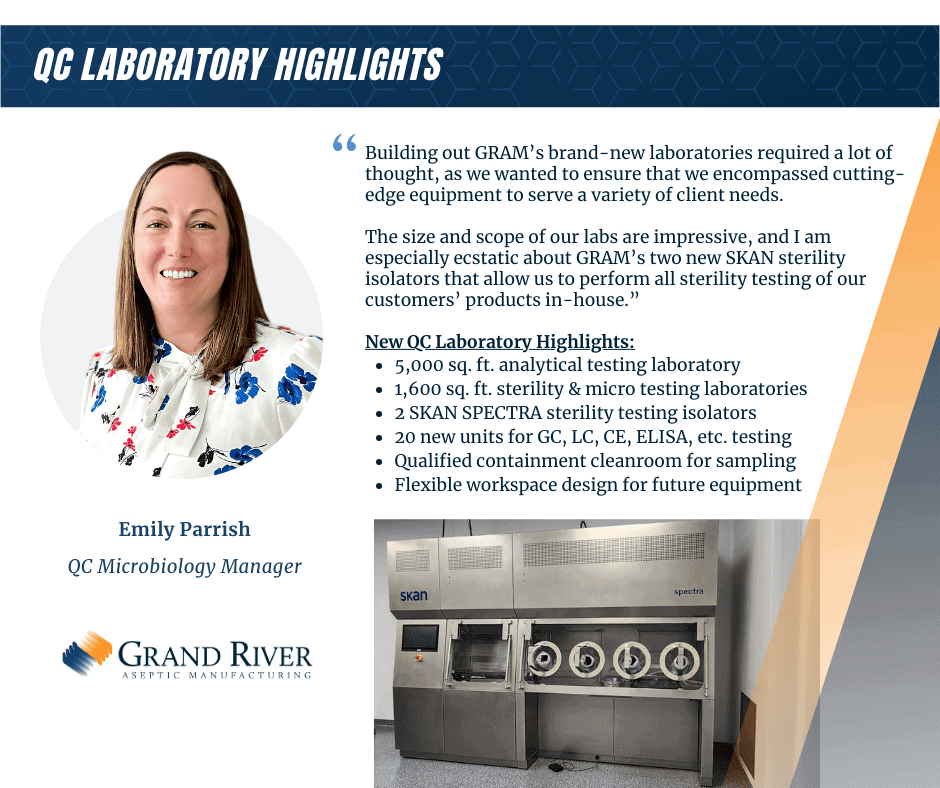
QC Analytical Laboratory
- QC analytical and sterility lab with SKAN sterility isolators to support lyophilized, biologics, and small molecule product testing
- Wet chemistry capabilities for Raw Material Testing
- Small-scale temperature control units for material/sample storage
- Additional large-scale temperature control units
- Oxygen Headspace Detection – Vials, Syringe and Cartridge
- Advanced LC Systems
- Advanced GC Systems