Sterile Filling Utilizing Innovative Technology Operated By Aseptic Manufacturing Experts
Liquid Vials, Lyophilized Vials, Syringes and Cartridges
Grand River Aseptic Manufacturing (GRAM) is a sterile injectable contract development and manufacturing organization (CDMO) with deep pharmaceutical manufacturing experience in biologics, small molecules, and vaccines. GRAM’s partnerships with global pharma and biotech leaders give our team development and manufacturing experience with a multitude of aseptic drug products.
GRAM’s aseptic filling facility encompasses top-of-the-line design elements with enhanced safety features and advanced equipment. The facility accommodates customer and industry-wide demand for cGMP parenteral drug development, manufacturing, analytical testing, and regulatory filing services for all batch sizes. GRAM delivers quality aseptic fill-finish services for clinical and commercial products.
Equipment
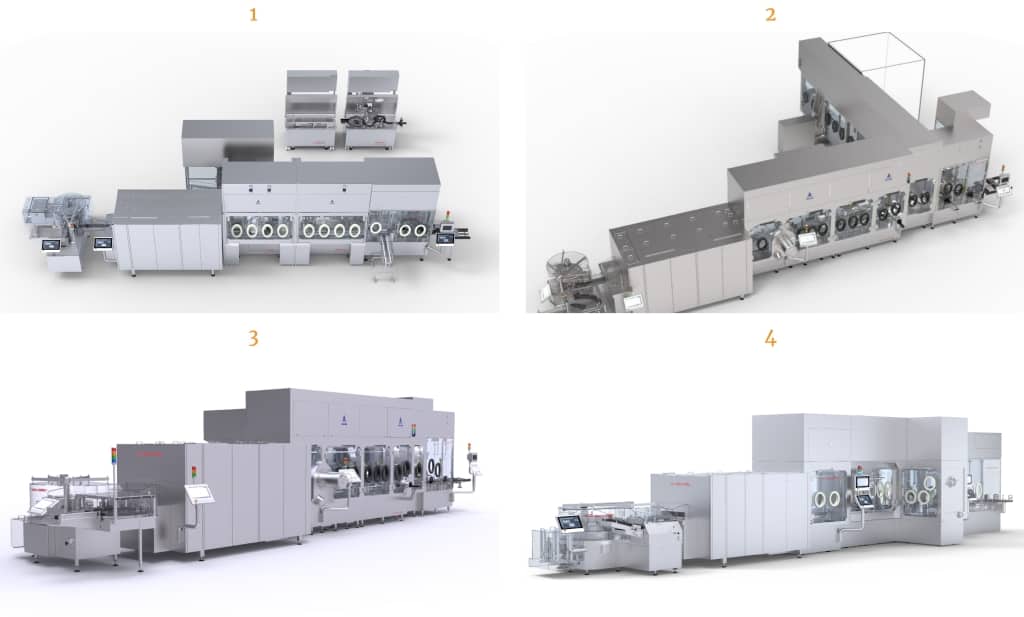
All four of our filling lines utilize identical filling and isolator technology, which allows our clients the ultimate versatility and flexibility to seamlessly scale their products as needed to succeed in the marketplace.
- Bausch+Ströbel VarioSys® modular vial and syringe/cartridge filler with SKAN Isolator
- Bausch+Ströbel high-speed 4-head vial filler with SKAN Isolator and IMA Lyophilizer
- Duplicate Bausch+Ströbel high-speed 4-head vial filler with SKAN Isolator
- Bausch+Ströbel 8-head vial filler with SKAN isolator
Capabilities
- Biologics, small molecule, and vaccines manufacturing
- Controlled substance manufacturing
- Formulation volumes up to 1,000 L
- Aseptic processing
- Low and high-speed filling
- Commercial-scale lyophilizer with auto-loader
- Single-use-systems or product-dedicated process equipment
Facility
- 2021 Facility of the Year Awards Winner for Special Recognition for Operational Agility: COVID-19 Impact
- 80,000 sq ft
- Three floors
- Three Grade C formulation suites
- Three Grade C filling rooms
- Methods Development, In-process & Micro Laboratories
- Walk-in frozen storage -20°C
- Walk-in cold storage +2°C to +8°C
- Customer viewing rooms